Afa chemi pharmaceutical co
The pharmaceutical company Afachemi began its operations in 1978 and, since 2007, has entered the field of producing oral and injectable antibiotics in accordance with international standards. Today, it is one of the key suppliers of both antibiotic and non-antibiotic medications in the country.
For many years, Afa Chemi has produced high-quality oral and injectable medicines by expanding modern production lines and adhering to GMP standards.
100+
High-quality products
Our company is constantly seeking improved methods to deliver the best quality in projects.
47+
Background and Experience
Since 1978, through the production of oral and injectable antibiotics, we have been one of the country’s leading pharmaceutical manufacturers.
8+
Pharmaceutical Production Line
Afachemi is equipped with more than eight pharmaceutical production lines compliant with the latest global standards.
17000+
Indirect customer
Today, Afachemi distributes its products across Iran through an extensive network of over 500 active customers and distributors.
Use of Modern Technologies
Afachemi؛ Combining Experience and Technology for a Healthier Tomorrow
At Afachemi, quality is never accidental. By utilizing the world’s most advanced pharmaceutical equipment and modern infrastructure, we have created a reliable path from production to treatment, delivering products worthy of the health of the Iranian people.
- Equipped with advanced Bosch machinery
- Fully automated production lines
- Specialized technology for producing sterile
- Modern infrastructure
- Quality assurance systems
- Advanced formulations
Our Products
- Oral
- 100mg
- Oral
- 75mg, 25mg
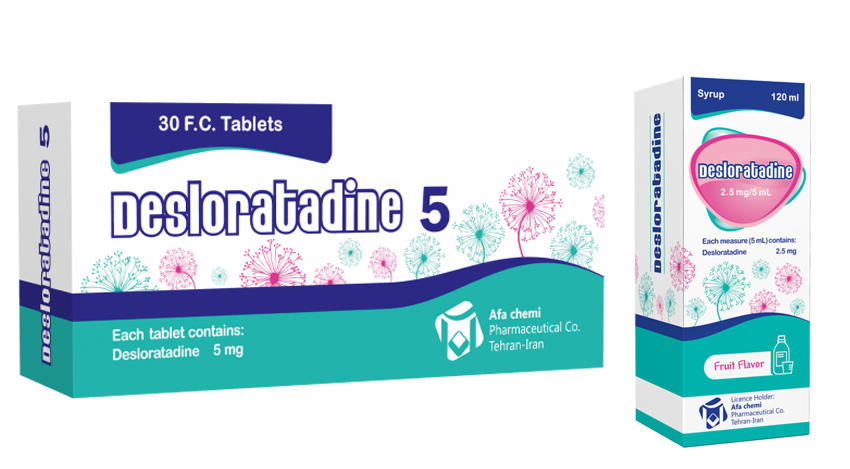
- Oral
- 2.5mg
All Projects of the Construction Company
You can view all the projects of our construction group on the company projects page.
Nophico Holding

Tehran

"From Iran to the World; with Nophico Holding"
Nophico Holding is one of Iran’s leading pharmaceutical groups, producing and distributing over 800 pharmaceutical products across more than 65 therapeutic categories. The holding actively participates in both public and private tenders, including hospitals and non-governmental organizations.
With a professional team in regulatory affairs, marketing, and distribution, and by adhering to GMP standards as well as WHO and P.I.C/S international requirements, Nophico has successfully registered and exported its products to international markets, including Central Asia, Africa, the Middle East, and neighboring countries, and continues to advance its global market development.
270+
Completed Projects
7+
Ongoing Project
98%
Resident Satisfaction Rate
Production Line
Certificates and Achievements of the Company
A Seal of Quality and Commitment


By implementing the most precise management and technical systems, we have earned the most reputable national and international certifications. Receiving standard marks from stringent European authorities and Iranian regulatory bodies guarantees that every product leaving our production lines has passed rigorous scientific and environmental scrutiny.
- 2023
- ISO 10004:2018
ISO 10004:2018 Management System Certificate
- 2022
- ISO 14001:2015
ISO 14001:2015 Environmental Management System Certificate
- 2022
- ISO 9001:2015
ISO 9001:2015 Quality Management System Certificate
- 2022
- IMS Certificate
Integrated Management System (IMS) Certificate
- 2024
- ISO 10015:2019
ISO 10015:2019 Management System Certificate
- 2022
- ISO 45001:2018
ISO 45001:2018 Management System Certificate
Our Company’s Expert Team





View All Specialists of Our Group
You can learn more about our group and our active specialists on the About Us page.
Latest News and Articles
Read the latest news and events of the country’s pharmaceutical industry on Afachemi’s company blog.
- 29 August 2025
- No Comments
- 29 August 2025
- No Comments
- 29 August 2025
- No Comments